Assessing CAR-T Cell Quality with Engineered Tissue Chips
Outcome/Accomplishment
The National Science Foundation (NSF)'s Engineering Research Center (ERC) for Cell Manufacturing Technologies (CMaT) has further evolved its designs for new tissue chips that rapidly assess chimeric antigen receptors (CAR) T-cell potency against multiple myeloma (MM) and glioblastoma cancers. CMaT researchers have demonstrated that a label-free glioblastoma cell coculture assay detects killing of target cancer cells by CAR T cells. The new findings build upon initial work that found genome-edited cells (NV) kill more quickly than the retroviral (RV) CAR T cells.
Impact/Benefits
CMaT's engineered tissue chips support more effective cancer immunotherapy applications. Genetically engineered CAR T-cell therapies show promise for treating MM and glioblastoma cancers by allowing for more specific recognition of antigens and T-cell signaling domains. The tissue cells developed by CMaT researchers will therefore help to assure more consistent, scalable, and low-cost production of high-quality living therapeutic cells for such cancers.
Explanation/Background
CMaT researchers Walsh and Mueller published their initial work on engineered tissue cells in Nature Biomedical Engineering in 2020. Their engineered tissue chips integrate label-free impedance and optical metabolic imaging (OMI)—a non-invasive, high-resolution, quantitative tool for improved monitoring of T cell activation and cytolysis. The cells are also compatible with next generation CRISPR-Cas9 genome-edited CAR T cell products and patientderived tumor cells.
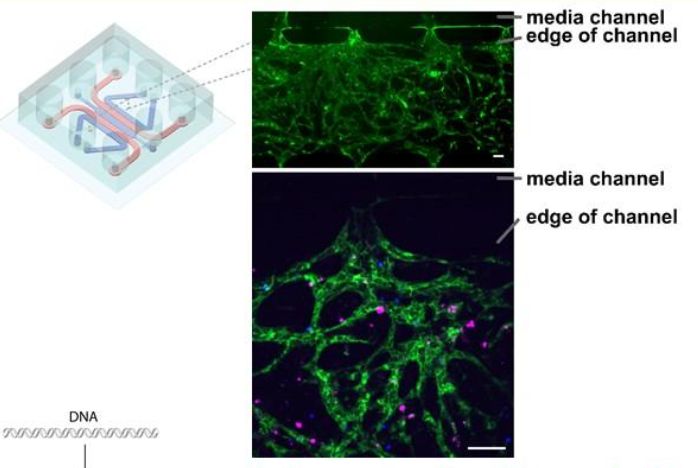
The team found that bone-marrow chips contain a permeable vascular network of endothelial cells (green) through which it is possible to flow through cells and other agents. It is also possible to co-culture primary MM cells (magenta) and CAR-T cells (blue) within the network. All scale bars are 100 microns (μm).
CMaT is a partnership of the University of Georgia, the University of Wisconsin-Madison, and the University of Puerto Rico, led by the Georgia Institute of Technology.
Location
Atlanta, GeorgiaStart Year
Advanced Manufacturing
Advanced Manufacturing
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
The National Science Foundation (NSF)'s Engineering Research Center (ERC) for Cell Manufacturing Technologies (CMaT) has further evolved its designs for new tissue chips that rapidly assess chimeric antigen receptors (CAR) T-cell potency against multiple myeloma (MM) and glioblastoma cancers. CMaT researchers have demonstrated that a label-free glioblastoma cell coculture assay detects killing of target cancer cells by CAR T cells. The new findings build upon initial work that found genome-edited cells (NV) kill more quickly than the retroviral (RV) CAR T cells.
Location
Atlanta, GeorgiaStart Year
Advanced Manufacturing
Advanced Manufacturing
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
CMaT's engineered tissue chips support more effective cancer immunotherapy applications. Genetically engineered CAR T-cell therapies show promise for treating MM and glioblastoma cancers by allowing for more specific recognition of antigens and T-cell signaling domains. The tissue cells developed by CMaT researchers will therefore help to assure more consistent, scalable, and low-cost production of high-quality living therapeutic cells for such cancers.
Explanation/Background
CMaT researchers Walsh and Mueller published their initial work on engineered tissue cells in Nature Biomedical Engineering in 2020. Their engineered tissue chips integrate label-free impedance and optical metabolic imaging (OMI)—a non-invasive, high-resolution, quantitative tool for improved monitoring of T cell activation and cytolysis. The cells are also compatible with next generation CRISPR-Cas9 genome-edited CAR T cell products and patientderived tumor cells.
The team found that bone-marrow chips contain a permeable vascular network of endothelial cells (green) through which it is possible to flow through cells and other agents. It is also possible to co-culture primary MM cells (magenta) and CAR-T cells (blue) within the network. All scale bars are 100 microns (μm).
CMaT is a partnership of the University of Georgia, the University of Wisconsin-Madison, and the University of Puerto Rico, led by the Georgia Institute of Technology.