Biomanufacturing Robust Vasculature Via Coaxial-Sacrificial Writing into Functional Tissues (co-SWIFT)
Outcome/Accomplishment
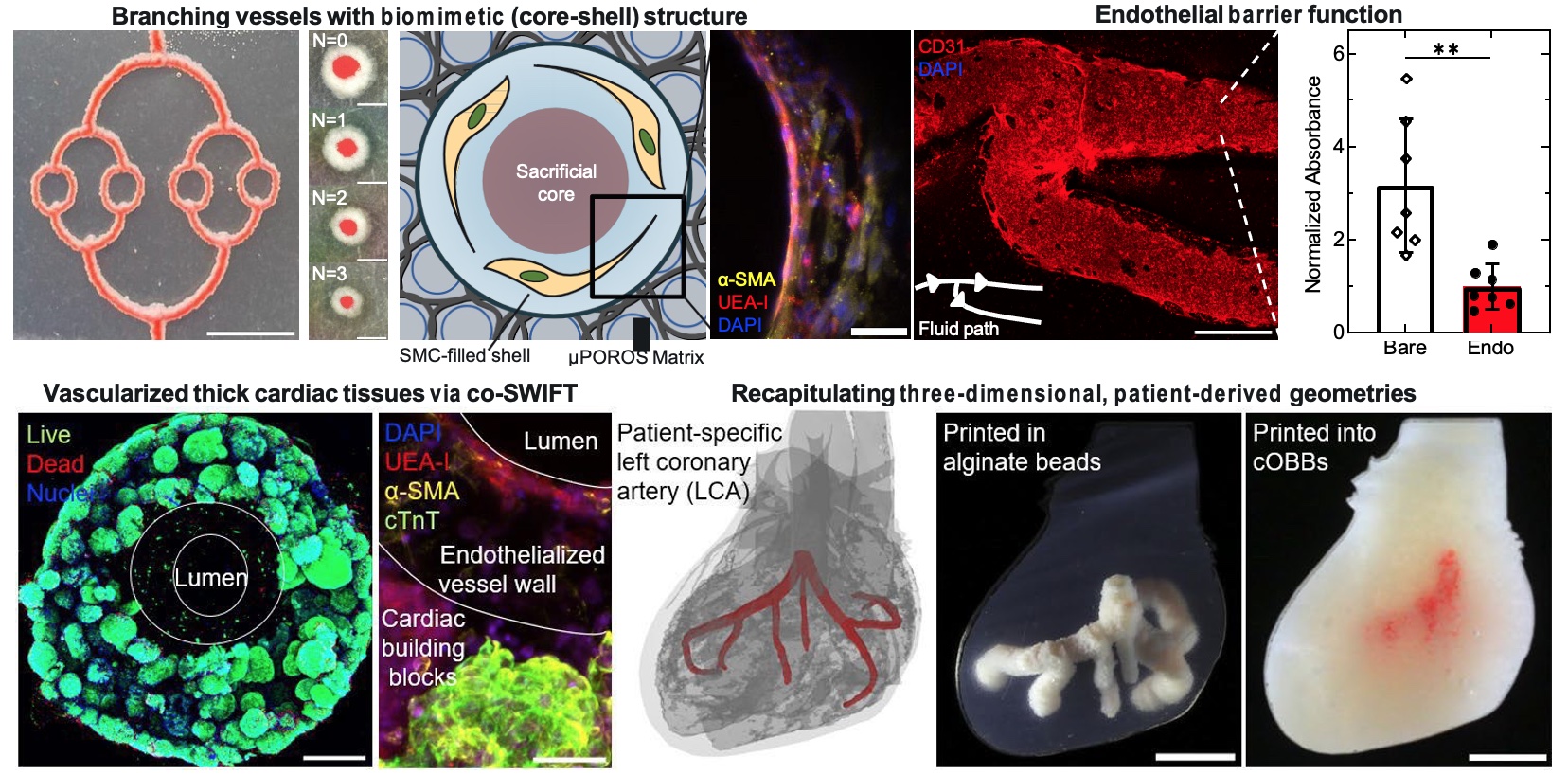
Researchers at the U.S. National Science Foundation (NSF)-funded Cellular Metamaterials (NSF CELL-MET) Engineering Research Center (ERC) based at Boston University (BU) developed a technique to embed functioning vessels into cardiac tissues via a technique called coaxial Sacrificial Writing Into Functional Tissues (co-SWIFT). Using a core-shell printhead that promotes interconnections between branching vessels, embedded biomimetic vessels are coaxially printed, possessing a smooth-muscle cell-laden shell that surrounds perfusable open spaces, allowing blood flow into engineered tissue.
Impact/Benefits
: More than seven million people die of heart disease annually. It is the number one cause of death in the United States and a leading cause of death worldwide. The advanced biomedical research at NSF CELL-MET aims to make it possible to repair damaged human heart tissue by leveraging innovations in stem cell biology, mechanobiology, materials science, and optics. In contrast to other printed human tissues that lack branching or multilayered architectures, NSF CELL-MET’s vascularized biomimetic cardiac tissues constructed using co-SWIFT have several important advantages. They mature under perfusion, beat synchronously, and exhibit a cardio-effective drug response in vitro. This advance opens new avenues for the scalable biomanufacturing of organ-specific tissues for drug testing, disease modeling, and therapeutic use.
Explanation/Background
Implantation in vivo serves as a key testbed for evaluating NSF CELL-MET’s engineered cardiac patches and their vascularization. A key milestone in benchmarking an engineered perfusable vasculature is the demonstration of its ability to deliver nutrients—most critically, oxygen—into a thick engineered tissue that supports survival of cells embedded in tissue. In contrast to the non-vascularized tissue that will exhibit significant cell death in inner tissue regions, vascularized tissues will maintain more than 90 percent cell viability.
The research team led by led by Paul Stankey, Sebastien Uzel, and Jennifer Lewis evolved a generalizable method for printing hierarchical branching vascular networks within soft and living matrices. The team leveraged both coaxial embedded 3-D printing (co-EMB3DP) as well as co-SWIFT to embed biomimetic vessels into granular hydrogel matrices and bulk cardiac tissues. Each method relies on an extended core-shell printhead that promotes facile interconnections between printed branching vessels. By optimizing multiple core-shell inks and matrices, the embedded biomimetic vessels can be coaxially printed. The vessels were seeded with a confluent layer of endothelial cells to exhibit good barrier function. The team is also recapitulating three-dimensional, patient-derived geometries. In that effort, biomimetic vascularized cardiac tissues were constructed of a densely cellular matrix of cardiac spheroids derived from human-induced pluripotent stem cells. This work resulted in a paper published in Biorxiv. The final manufacturing milestone will be to generate a centimeter-scale cardiac patch with embedded perfusable vasculature that maintains stable function (as measured by tissue viability, structure, and electromechanical function.)
Location
Boston, MassachusettsStart Year
Biotechnology and Healthcare
Biotechnology and Healthcare
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
Researchers at the U.S. National Science Foundation (NSF)-funded Cellular Metamaterials (NSF CELL-MET) Engineering Research Center (ERC) based at Boston University (BU) developed a technique to embed functioning vessels into cardiac tissues via a technique called coaxial Sacrificial Writing Into Functional Tissues (co-SWIFT). Using a core-shell printhead that promotes interconnections between branching vessels, embedded biomimetic vessels are coaxially printed, possessing a smooth-muscle cell-laden shell that surrounds perfusable open spaces, allowing blood flow into engineered tissue.
Location
Boston, MassachusettsStart Year
Biotechnology and Healthcare
Biotechnology and Healthcare
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
: More than seven million people die of heart disease annually. It is the number one cause of death in the United States and a leading cause of death worldwide. The advanced biomedical research at NSF CELL-MET aims to make it possible to repair damaged human heart tissue by leveraging innovations in stem cell biology, mechanobiology, materials science, and optics. In contrast to other printed human tissues that lack branching or multilayered architectures, NSF CELL-MET’s vascularized biomimetic cardiac tissues constructed using co-SWIFT have several important advantages. They mature under perfusion, beat synchronously, and exhibit a cardio-effective drug response in vitro. This advance opens new avenues for the scalable biomanufacturing of organ-specific tissues for drug testing, disease modeling, and therapeutic use.
Explanation/Background
Implantation in vivo serves as a key testbed for evaluating NSF CELL-MET’s engineered cardiac patches and their vascularization. A key milestone in benchmarking an engineered perfusable vasculature is the demonstration of its ability to deliver nutrients—most critically, oxygen—into a thick engineered tissue that supports survival of cells embedded in tissue. In contrast to the non-vascularized tissue that will exhibit significant cell death in inner tissue regions, vascularized tissues will maintain more than 90 percent cell viability.
The research team led by led by Paul Stankey, Sebastien Uzel, and Jennifer Lewis evolved a generalizable method for printing hierarchical branching vascular networks within soft and living matrices. The team leveraged both coaxial embedded 3-D printing (co-EMB3DP) as well as co-SWIFT to embed biomimetic vessels into granular hydrogel matrices and bulk cardiac tissues. Each method relies on an extended core-shell printhead that promotes facile interconnections between printed branching vessels. By optimizing multiple core-shell inks and matrices, the embedded biomimetic vessels can be coaxially printed. The vessels were seeded with a confluent layer of endothelial cells to exhibit good barrier function. The team is also recapitulating three-dimensional, patient-derived geometries. In that effort, biomimetic vascularized cardiac tissues were constructed of a densely cellular matrix of cardiac spheroids derived from human-induced pluripotent stem cells. This work resulted in a paper published in Biorxiv. The final manufacturing milestone will be to generate a centimeter-scale cardiac patch with embedded perfusable vasculature that maintains stable function (as measured by tissue viability, structure, and electromechanical function.)