Functional Materials for Selective Ion Transport
Outcome/Accomplishment
Researchers with the Nanotechnology Enabled Water Treatment (NEWT) Engineering Research Center (ERC) have made significant advances in the development of functionalized polymers, covalent organic frameworks (COFs), and metal organic frameworks (MOFs) for ion-selective separations; very high selectivity for copper (Cu) transport over other divalent ions (such as magnesium (Mg) and calcium (Ca)) has been demonstrated using multilayer polymer films. The findings demonstrate the potential for these new materials for selective ion transport. The National Science Foundation (NSF)-sponsored ERC is based at Rice University.
Impact/Benefits
To maintain realistic feed waters, unprecedented selectivity is needed for the removal of challenging groundwater contaminants and scale-forming species prior to desalination. NEWT's work develops new, highly selective materials that can be incorporated into continuous treatment processes for the removal of scale-forming ions. By selectively separating scale-forming ions prior to desalination, membrane scaling is reduced, and the performance and reliability of the subsequent reverse osmosis stage can be enhanced. The goal of this work is to enable low-energy, low-cost, chemical-free desalination methods; however, a fundamental understanding of molecular recognition could also be applied to address target contaminant (nitrate (NO3-)) removal from drinking water and fit-for-purpose treatment of wastewater.
Explanation/Background
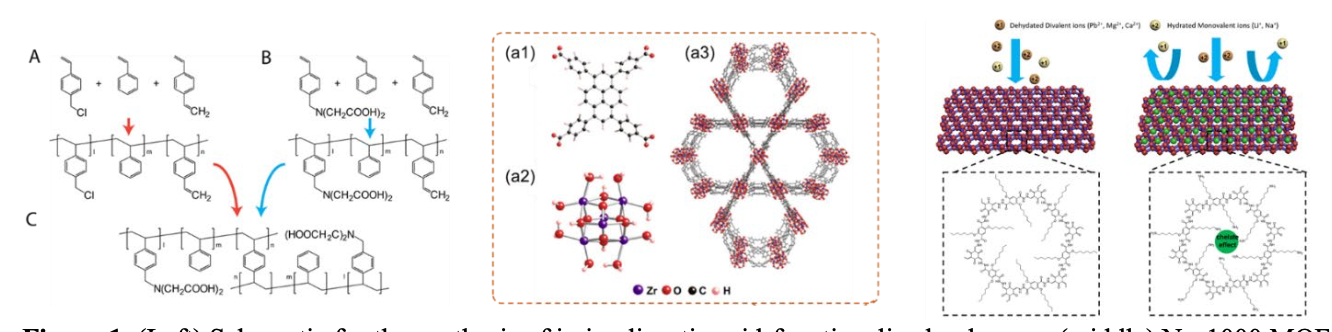
The molecular basis for selective ion transport is poorly understood. NEWT's research explores the mechanisms by which materials can discriminate between similar ionic species. The team hypothesized that molecular binding sites that can selectively remove water shells from ions (as seen in some biological channels), combined with precisely tailored pore/channel morphology, can enable higher selectivity.
Chemically tailored polymers, COFs, and MOFs were created with unique combinations of nanopore morphology and high-affinity chemical moieties. The team's designs used host guest chemistries in synthetic materials and the bottom-up design of materials with precise control of pore size and binding sites. Then the materials were incorporated into ion-exchange membranes (IEMs) and coatings capable of selectively transporting target ions. Finally, the membranes and materials were applied in electrosorption and electrodialysis processes for selective ion removal. The results were studied to optimize selectivity under realistic solution chemistry and operating conditions. In studying the results, the researchers quantified the cation/anion perm-selectivity of the membranes developed and screened ion transport and selectivity as a function of electric field, membrane thickness, feed salinity, and in both single-ion and mixed-ion solutions.
Successful results included produced polymers functionalized with polystyrene sulfonate or iminodiacetate capable of selective binding towards divalent ions. The ion selectivity of polymer membranes is strongly controlled by ion-membrane binding strength. Strong selectivity for the transport of metallic ions (Cu2+) over monovalent ions was demonstrated. MOFs capable of chromate adsorption and reduction were also developed. Results suggest that increasing ion-membrane interactions may provide selectivity for scale-forming divalent ions in polymeric membranes.
Location
Houston, Texaswebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
Researchers with the Nanotechnology Enabled Water Treatment (NEWT) Engineering Research Center (ERC) have made significant advances in the development of functionalized polymers, covalent organic frameworks (COFs), and metal organic frameworks (MOFs) for ion-selective separations; very high selectivity for copper (Cu) transport over other divalent ions (such as magnesium (Mg) and calcium (Ca)) has been demonstrated using multilayer polymer films. The findings demonstrate the potential for these new materials for selective ion transport. The National Science Foundation (NSF)-sponsored ERC is based at Rice University.
Location
Houston, Texaswebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
To maintain realistic feed waters, unprecedented selectivity is needed for the removal of challenging groundwater contaminants and scale-forming species prior to desalination. NEWT's work develops new, highly selective materials that can be incorporated into continuous treatment processes for the removal of scale-forming ions. By selectively separating scale-forming ions prior to desalination, membrane scaling is reduced, and the performance and reliability of the subsequent reverse osmosis stage can be enhanced. The goal of this work is to enable low-energy, low-cost, chemical-free desalination methods; however, a fundamental understanding of molecular recognition could also be applied to address target contaminant (nitrate (NO3-)) removal from drinking water and fit-for-purpose treatment of wastewater.
Explanation/Background
The molecular basis for selective ion transport is poorly understood. NEWT's research explores the mechanisms by which materials can discriminate between similar ionic species. The team hypothesized that molecular binding sites that can selectively remove water shells from ions (as seen in some biological channels), combined with precisely tailored pore/channel morphology, can enable higher selectivity.
Chemically tailored polymers, COFs, and MOFs were created with unique combinations of nanopore morphology and high-affinity chemical moieties. The team's designs used host guest chemistries in synthetic materials and the bottom-up design of materials with precise control of pore size and binding sites. Then the materials were incorporated into ion-exchange membranes (IEMs) and coatings capable of selectively transporting target ions. Finally, the membranes and materials were applied in electrosorption and electrodialysis processes for selective ion removal. The results were studied to optimize selectivity under realistic solution chemistry and operating conditions. In studying the results, the researchers quantified the cation/anion perm-selectivity of the membranes developed and screened ion transport and selectivity as a function of electric field, membrane thickness, feed salinity, and in both single-ion and mixed-ion solutions.
Successful results included produced polymers functionalized with polystyrene sulfonate or iminodiacetate capable of selective binding towards divalent ions. The ion selectivity of polymer membranes is strongly controlled by ion-membrane binding strength. Strong selectivity for the transport of metallic ions (Cu2+) over monovalent ions was demonstrated. MOFs capable of chromate adsorption and reduction were also developed. Results suggest that increasing ion-membrane interactions may provide selectivity for scale-forming divalent ions in polymeric membranes.