New Microscope Technology for Studying Cardiac Tissue
Outcome/Accomplishment
Over the past two decades, there has been an increased interest in the development of microscopy systems to monitor cellular activity over large three-dimensional fields of view with high resolution as a function of time. Researchers at Boston University have developed and demonstrated scanning microscope technology for performing high speed imaging of cardiac tissue, advancing the field of personalized treatment of heart disease. This research is being supported by the National Science Foundation (NSF)-funded Engineering Research Center (ERC) in Cellular Metamaterials (CELL-MET), headquartered at BU.
Impact/Benefits
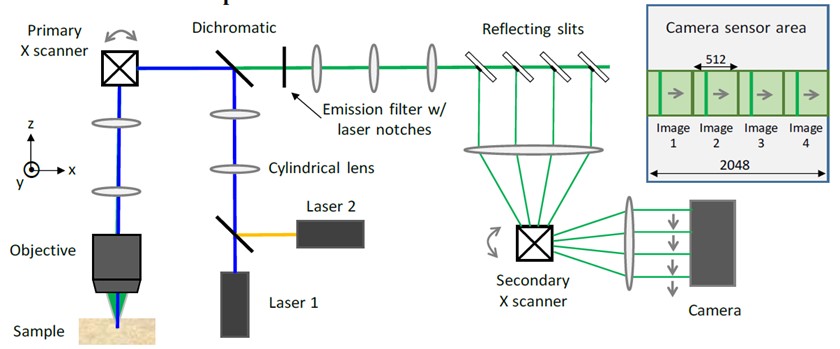
This advanced technique—called line-scan multi-z confocal microscopy—overcomes technical challenges in prior state-of-the-art technology. Light travels through the sample under a conventional microscope as far into the specimen as it can penetrate, while a confocal microscope focuses a smaller beam of light at one narrow depth level at a time, thus achieving a controlled and highly focused depth of field. Line scan imaging uses a single line of sensor pixels (effectively one-dimensional) to build up a two-dimensional image. For a given field of view, one line scan camera typically provides more resolution than multiple area scan cameras, at a lower cost, without image smear or the redundant processing of frame overlaps. Z-stacking (also known as focus stacking) is a digital image processing method which combines multiple images taken at different focal distances to provide a composite image with a greater depth of field (i.e. the thickness of the plane of focus) than any of the individual source images.
Explanation/Background
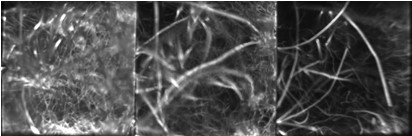
Fast, volumetric imaging over large scales has been a long-standing goal in biological microscopy and will be critical for the pursuit of voltage imaging in heart tissue. Scanning techniques such as fluorescence confocal microscopy can acquire 2D images at high resolution and high speed but extending the acquisition to multiple planes at different depths requires an axial scanning mechanism that drastically reduces the acquisition speed. To address this challenge, the CELL-MET researchers developed their augmented variant of confocal microscopy, where the key innovation consists of using a series of reflecting pinholes axially distributed in the detection plane, each one probing a different depth within the sample. As no axial scanning mechanism is involved, the new technique provides simultaneous multiplane imaging over fields of view larger than a millimeter.
Location
Boston, MassachusettsStart Year
Biotechnology and Healthcare
Biotechnology and Healthcare
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
Over the past two decades, there has been an increased interest in the development of microscopy systems to monitor cellular activity over large three-dimensional fields of view with high resolution as a function of time. Researchers at Boston University have developed and demonstrated scanning microscope technology for performing high speed imaging of cardiac tissue, advancing the field of personalized treatment of heart disease. This research is being supported by the National Science Foundation (NSF)-funded Engineering Research Center (ERC) in Cellular Metamaterials (CELL-MET), headquartered at BU.
Location
Boston, MassachusettsStart Year
Biotechnology and Healthcare
Biotechnology and Healthcare
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
This advanced technique—called line-scan multi-z confocal microscopy—overcomes technical challenges in prior state-of-the-art technology. Light travels through the sample under a conventional microscope as far into the specimen as it can penetrate, while a confocal microscope focuses a smaller beam of light at one narrow depth level at a time, thus achieving a controlled and highly focused depth of field. Line scan imaging uses a single line of sensor pixels (effectively one-dimensional) to build up a two-dimensional image. For a given field of view, one line scan camera typically provides more resolution than multiple area scan cameras, at a lower cost, without image smear or the redundant processing of frame overlaps. Z-stacking (also known as focus stacking) is a digital image processing method which combines multiple images taken at different focal distances to provide a composite image with a greater depth of field (i.e. the thickness of the plane of focus) than any of the individual source images.
Explanation/Background
Fast, volumetric imaging over large scales has been a long-standing goal in biological microscopy and will be critical for the pursuit of voltage imaging in heart tissue. Scanning techniques such as fluorescence confocal microscopy can acquire 2D images at high resolution and high speed but extending the acquisition to multiple planes at different depths requires an axial scanning mechanism that drastically reduces the acquisition speed. To address this challenge, the CELL-MET researchers developed their augmented variant of confocal microscopy, where the key innovation consists of using a series of reflecting pinholes axially distributed in the detection plane, each one probing a different depth within the sample. As no axial scanning mechanism is involved, the new technique provides simultaneous multiplane imaging over fields of view larger than a millimeter.