NEWT-NERC Student Discovers Novel Chemical Compounds Activated by Sunlight That Destroy Toxic PFAS
Outcome/Accomplishment
Bo Wang, a Ph.D. student in Chemical Engineering at Rice University (RU) working with the National Science Foundation (NSF)-funded Nanosystems Engineering Research Center (NERC) for Nanotechnology Enabled Water Treatment (NEWT), has discovered novel chemical compounds capable of destroying polyfluoroalkyl substances (PFAS), the toxic chemicals found in our bodies due to the prevalence of plastics in our water supply. Specifically, Wang designed an ultraviolet UV-A light-powered catalyst that breaks down perfluorooctanoic acid (PFOA, a type of PFAS) fifteen times more rapidly than prior solutions. Wang has been named to the inaugural class of Rice Innovation Fellows as a result of this work. The program is a concerted effort to translate breakthrough innovations emerging from research directly into promising new commercial ventures.
Impact/Benefits
Since the 1930s PFAS has been used in hundreds of products including stain- and water-resistant fabrics and carpeting, cleaning products, paints, fire-fighting foams, cookware, food packaging, and food processing equipment. While the Food and Drug Administration (FDA) has authorized the use of PFAS for limited use, it acknowledges that these "forever chemical" compounds feature a strong, carbon-fluorine bond which does not degrade easily. As a result, widespread use of PFAS across a range of products persists in the environment from past and current use, resulting in increasing levels of contamination of the air, water, and soil. The U.S. Centers for Disease Control and Prevention (CDC) reports that PFAS exposure poses a range of human health risks, including cancer, liver damage, decreased fertility, and increased risk of asthma and thyroid disease. In March 2021, the Environmental Protection Agency (EPA) announced plans to develop Federal standards to limit PFOA contamination in drinking water. Wong's research discovery provides a viable, cost-effective solution for directly reducing the amount of PFOA contaminants found in water systems.
Explanation/Background
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a class of man-made chemicals not found naturally in the environment. Of these chemicals, PFOA (sometimes known as "C8") and PFOS are the two PFAS that have been the most extensively produced and studied.
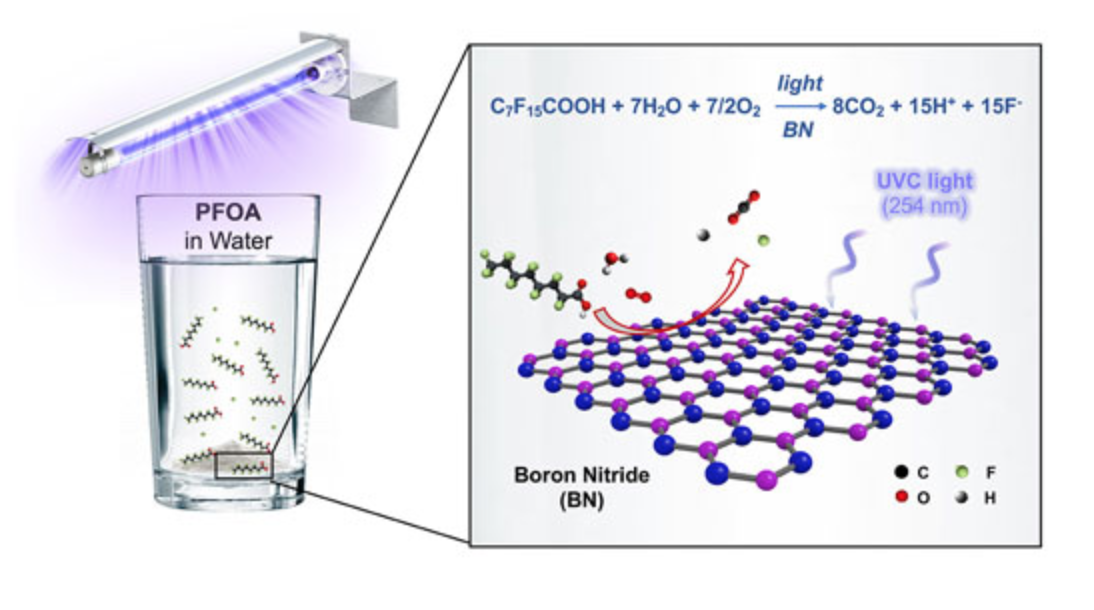
In 2020, a NEWT research team led by Michael Wong found that boron nitride, a commercially available powder commonly used in cosmetics, could destroy 99% of PFOA in water samples within just a few hours when it was exposed to UV light with a wavelength of 254 nanometers. However, boron nitride is activated by short-wave UV light which is generally filtered out by the atmosphere. In contrast, titanium dioxide, commonly found in sunscreen, is activated by long-wave UV light (or UV-A) and can also breakdown PFOA, but at a slower pace.
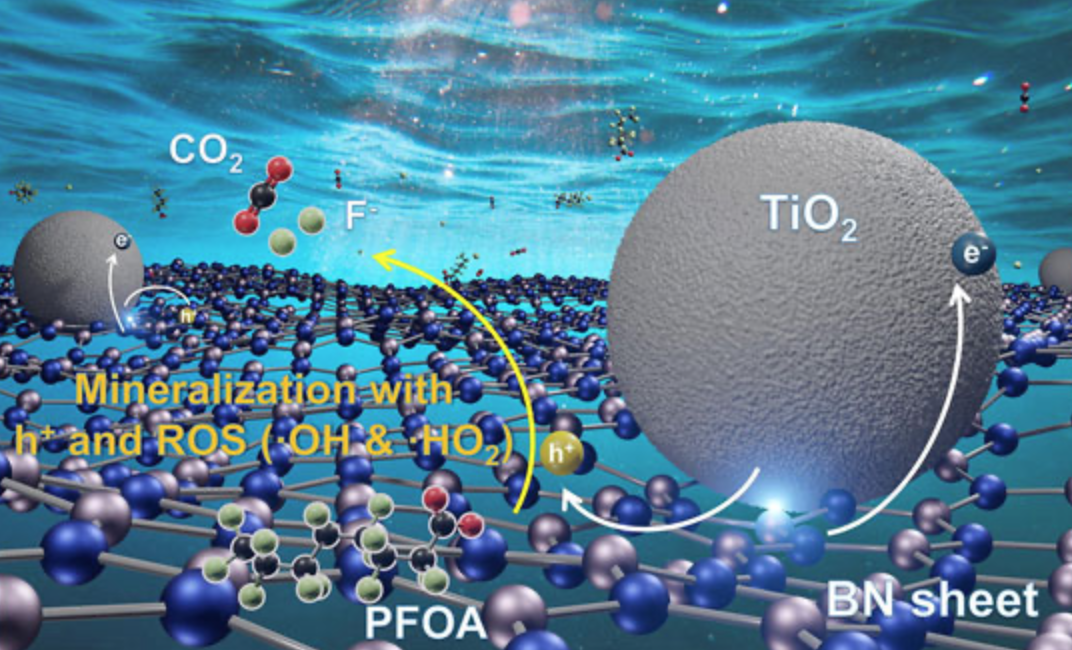
Wang's work leverages the best features of these two catalysts to create a UV-A powered composite that destroys PFOA 15 times faster than prior solutions. In outdoor experiments using plastic water bottles under natural sunlight, they found the boron nitride-titanium dioxide composites could degrade about 99% of PFOA in deionized water in less than three hours. The process took about nine hours in salty water.
As a Rice Innovation Fellow, Wang receives educational and financial support for pursuing a next-generation scientist- and engineer-led spinout venture based on this research. The program offers equity-free funding, a coworking space, and a yearlong program of education and personalized mentorship through the Liu Idea Lab for Innovation & Entrepreneurship (Lilie Lab).
Location
Houston, Texaswebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
Bo Wang, a Ph.D. student in Chemical Engineering at Rice University (RU) working with the National Science Foundation (NSF)-funded Nanosystems Engineering Research Center (NERC) for Nanotechnology Enabled Water Treatment (NEWT), has discovered novel chemical compounds capable of destroying polyfluoroalkyl substances (PFAS), the toxic chemicals found in our bodies due to the prevalence of plastics in our water supply. Specifically, Wang designed an ultraviolet UV-A light-powered catalyst that breaks down perfluorooctanoic acid (PFOA, a type of PFAS) fifteen times more rapidly than prior solutions. Wang has been named to the inaugural class of Rice Innovation Fellows as a result of this work. The program is a concerted effort to translate breakthrough innovations emerging from research directly into promising new commercial ventures.
Location
Houston, Texaswebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
Since the 1930s PFAS has been used in hundreds of products including stain- and water-resistant fabrics and carpeting, cleaning products, paints, fire-fighting foams, cookware, food packaging, and food processing equipment. While the Food and Drug Administration (FDA) has authorized the use of PFAS for limited use, it acknowledges that these "forever chemical" compounds feature a strong, carbon-fluorine bond which does not degrade easily. As a result, widespread use of PFAS across a range of products persists in the environment from past and current use, resulting in increasing levels of contamination of the air, water, and soil. The U.S. Centers for Disease Control and Prevention (CDC) reports that PFAS exposure poses a range of human health risks, including cancer, liver damage, decreased fertility, and increased risk of asthma and thyroid disease. In March 2021, the Environmental Protection Agency (EPA) announced plans to develop Federal standards to limit PFOA contamination in drinking water. Wong's research discovery provides a viable, cost-effective solution for directly reducing the amount of PFOA contaminants found in water systems.
Explanation/Background
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a class of man-made chemicals not found naturally in the environment. Of these chemicals, PFOA (sometimes known as "C8") and PFOS are the two PFAS that have been the most extensively produced and studied.
In 2020, a NEWT research team led by Michael Wong found that boron nitride, a commercially available powder commonly used in cosmetics, could destroy 99% of PFOA in water samples within just a few hours when it was exposed to UV light with a wavelength of 254 nanometers. However, boron nitride is activated by short-wave UV light which is generally filtered out by the atmosphere. In contrast, titanium dioxide, commonly found in sunscreen, is activated by long-wave UV light (or UV-A) and can also breakdown PFOA, but at a slower pace.
Wang's work leverages the best features of these two catalysts to create a UV-A powered composite that destroys PFOA 15 times faster than prior solutions. In outdoor experiments using plastic water bottles under natural sunlight, they found the boron nitride-titanium dioxide composites could degrade about 99% of PFOA in deionized water in less than three hours. The process took about nine hours in salty water.
As a Rice Innovation Fellow, Wang receives educational and financial support for pursuing a next-generation scientist- and engineer-led spinout venture based on this research. The program offers equity-free funding, a coworking space, and a yearlong program of education and personalized mentorship through the Liu Idea Lab for Innovation & Entrepreneurship (Lilie Lab).