It is important to work closely with the institutional sponsored programs offices to manage this large, complex cooperative agreement with many academic and industrial partners. ERCs push the envelope in regards to research, technology transfer, education and outreach, and administration as well. The AD should take the time to meet with the people who help manage proposals and awards and understand their roles in order to learn how to move things through the system. In addition, the AD should do the same with administrative staff at partner institutions, since most people feel excited and proud to be part of such a dynamic program. The AD will create a “win-win” situation by integrating administrative personnel into the project and inviting them to meetings and reviews. The “Authorized Organizational Representative” (AOR) is usually the director of the institutional sponsored programs office, and the AD will interact with her/him throughout the life of the center. The goal is to fulfill the terms of the cooperative agreement while adhering to laws and rules of federal, state and local government, the academic institutions, the ERC program, and the NSF – this is not always straightforward. Understanding the rules and keeping good records is a shared responsibility between the lead institution and the academic partners.
Tip: Regular teleconferences with financial and sponsored programs staff at each partner institution can facilitate communication and minimize misunderstandings and problems.
6.2.1 Award Management Resources
It can be confusing to know where to look for information or guidance. The AD should become familiar with key award management reference documents and websites.
- ERC Library – This website is maintained by the developer (ICF International) of the ERCWeb Annual Report Data Entry System. The library contains Guidelines for Preparing Annual Reports and Renewal Proposals, Guidelines for ERCWeb Data Entry, Annual and Renewal Site Visit Guidelines, Performance Review Criteria and Protocol, Glossary of terms, and other useful ERC program specific documentation. https://www.erc-reports.org/public/library
- ERC Association website -This website is a resource for all those involved in the NSF ERC program. It contains information on the program, the individual centers, and their achievements, research initiatives, innovation ecosystem development, education programs, and the ERC Best Practices Manual.http:/erc-assoc.org
- Cooperative Agreement – Each center’s official cooperative agreement with NSF can be found on the NSF Fastlane website under the Principal Investigator’s login. https://www.fastlane.nsf.gov/
- NSF Award Conditions http://www.nsf.gov/awards/managing/award_conditions.jsp?org=NSF
- Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards2 CFR Chapter I, and Chapter II, Parts 200, 215, 220, 225, and 230 . This “omni-circular” or “supercircular” consolidates the regulations of eight OMB circulars (A-21, A-50, A-87, A-89, A-102, A-110, A-122, A-133) into one uniform set of regulations for all grant recipients. Effective December 26, 2014. http://www.ecfr.gov/cgi-bin/text-idx?SID=1d6de4ac49815c17087194eb72498042&tpl=/ecfrbrowse/Title02/2cfr200_main_02.tpl
- Proposal and Award Policies and Procedures Guide (includes Grant Proposal Guide (GPG) and Award and Administration Guide (AAG) http://www.nsf.gov/publications/pub_summ.jsp?ods_key=nsf14001&org=NSF
Circulars which will be replaced by the super-circular on December 26, 2014:
Key Definitions
Authorized Organizational Representative (AOR)/authorized representative – The administrative official who, on behalf of the proposing organization, is empowered to make certifications and assurances and can commit the organization to the conduct of a project that NSF is being asked to support as well as adhere to various NSF policies and grant requirements. http://www.nsf.gov/pubs/policydocs/pappguide/nsf14001/index.jsp#definitions
Cooperative Agreement – Type of assistance award which should be used when substantial agency involvement is anticipated during the project performance period. Substantial agency involvement may be necessary when an activity is technically and/or managerially complex and requires extensive or close coordination between NSF and the awardee. Examples of projects which might be suitable for cooperative agreements if there will be substantial agency involvement are: research centers, large curriculum projects, multi-user facilities, projects which involve complex subcontracting, construction or operations of major in-house university facilities, and major instrumentation development. http://www.nsf.gov/pubs/policydocs/pappguide/nsf14001/index.jsp#definitions
6.2.2 Compliance Decisions
Official guidance on grant compliance is contained in the Office of Management and Budget publication, 2 CFR Chapter I, and Chapter II, Parts 200, 215, 220, 225, and 230 Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards. This “omni-circular” or “supercircular” consolidates the regulations of eight OMB circulars (A-21, A-50, A-87, A-89, A-102, A-110, A-122, A-133) into one uniform set of regulations.
Figure 2 illustrates the many factors involved in making grant management decisions The NSF Cooperative Agreement is the ruling document for each center, but this document must be considered in relation to institutional policies and public laws, as well as the ERC Program Rules, the NSF Agency Terms and Conditions, and the OMB Circulars. It is always smart to document the reasoning behind complex decisions as the center works to establish clear and consistent policies.
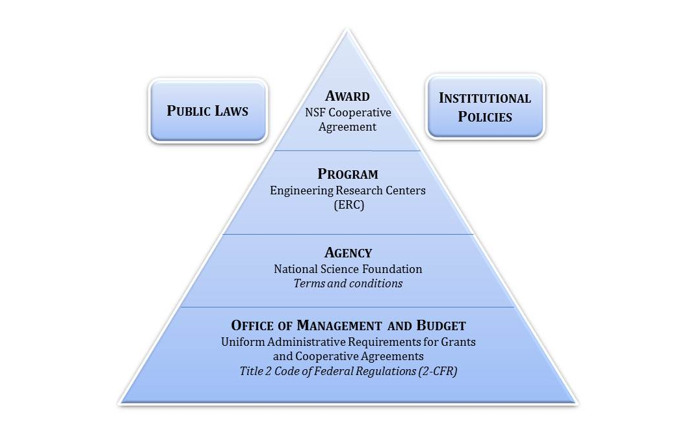
Figure 2. Compliance Diagram
6.2.3 General Cost Principles for Educational Institutions
Academic institutions will have procedures and policies in place to ensure that all costs charged to federal grants are allowable, allocable, and reasonable.
- Allowable – must meet the sponsor’s definition of categories permissible to be charged to the project it funds
- Allocable – costs must be charged to a project in proportion to the benefit received
- Reasonable – the action that a prudent person would have taken at the time the decision to incur the cost was made.
This determination can be complicated however, especially since an ERC is charged with creating an innovative innovation ecosystem and must interact with industry and a Student Leadership Council in non-traditional ways. It can be challenging to characterize the various types of support such as grants, industrial membership fees, donations, gifts, and other contributions. Document the decisions and reasoning and be as consistent as possible.
6.2.4 Audits of Federal Awards
Annual audits of federal awards are conducted at each academic institution, and the NSF ERC program award may be selected for audit at any time. Expenditures on federal awards must comply with the Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards. Always check institutional rules and work with local accounting offices to monitor compliance; but some high-level guidance is provided below:
Mandatory Cost Share and Matching Funds Information – Closely monitor compliance with mandatory cost sharing obligations stated in the cooperative agreement and keep documentation. Committed cost share effort should also be tracked. Note that industrial membership fees are considered program income generated as a result of a federally sponsored project, making program income unallowable as eligible cost sharing
Sub-recipient Monitoring – PIs are required to monitor the activities of sub-recipients to ensure that performance goals included in the subaward are achieved and cost share commitments are documented. All sub-recipient invoices must be approved and signed by the PI attesting that charges are consistent with the scope of work and within the approved budget for the sub-recipient.
Cost Transfers and Timeliness of Charges – All charges and cost transfers should be processed within 90 days of the original transaction. Transfers exceeding the 90 day limit will usually require a detailed justification. All cost transfers require explanation of the original error and justification of transfer to the grant.
Administrative and Clerical Salaries – The salaries of administrative and clerical staff are not normally directly charged to a federal grant, but direct charging of these costs is allowed for major projects such as an ERC if the budget explicitly provides funds for administrative or clerical staff to complete specific tasks. New guidance suggests that administrative support that is integral to the project may be allowable.
Administrative Supplies and General Purpose Equipment – Administrative supplies (copy paper, toner, bottled water, etc.) and general purpose equipment (desktops and laptops, cell phone charges, fax machines, and copiers) are not normally directly charged to a federal grant unless there is a specific requirement in the grant for these items and the items are used primarily and directly for the project.
Subsequent Changes in Level of Effort from Proposal – PIs are required to notify NSF if their percentage of effort changes significantly from the level specified in the proposal.
Program Income – PIs should ensure that all program income is properly calculated, recorded and expended in accordance with program requirements.
Disclaimers and Acknowledgments Contained in Publications – PIs should ensure that all publications and presentations include proper disclaimers and acknowledgments of NSF support.
See Attachment 6.4 – Sample NSF Acknowledgment Language
Timely Filing of Progress and Technical Reports – PIs should ensure that the Annual Report is submitted to the NSF Program Officer, to Fastlane and Research.gov, and that print and CD copies are mailed to NSF by the required due date.
Disposition and Transfers of Equipment – Be sure to track the purchase, transfer, and disposal of all equipment. Equipment transfers to other institutions, changes in location and disposals of equipment need to be authorized and processed correctly according to the rules of the institution.
6.2.5 Responsible Conduct of Research (RCR)
Each academic institution is required to ensure that research is conducted in an ethical manner. Determine the institutional requirements and set up a plan to facilitate compliance. This can entail an online training program or a simple signed acknowledgement, but it is specific to each institution. Refer to Section 7009 of the “America Creating Opportunities to Meaningfully Promote Excellence in Technology, Education, and Science (COMPETES) Act (42 U.S.C. 18620-1).” This section of the Act requires that “each institution that applies for financial assistance from the Foundation for science and engineering research or education describe in its grant proposal a plan to provide appropriate training and oversight in the responsible and ethical conduct of research to undergraduate students, graduate students, and postdoctoral researchers participating in the proposed research project.”http://www.nsf.gov/bfa/dias/policy/rcr.jsp
6.2.6 Protection of Human Subjects
Each academic institution in the ERC will need to adhere to the Code of Federal Regulations for the Protection of Human Subjects (45 CFR 46)
There is usually an office on campus responsible for monitoring protection of human subjects and the center research may require approval of an Institutional Review Board (IRB). The experts on each campus can offer guidance to ensure compliance.
The NSF supports research involving human subjects when the project has been certified by a responsible body to be in compliance with the federal government's "Common Rule" for the protection of human subjects. The official NSF version of Code of Federal Regulations 45 CFR 690.101-124 is available at http://www.nsf.gov/bfa/dias/policy/docs/45cfr690.pdf. The regulations give grantee institutions the responsibility for setting up "Institutional Review Boards" (IRBs) to review research protocols and designs and ensure the protection of the rights of human subjects. http://www.nsf.gov/bfa/dias/policy/human.jsp
6.2.7 Effort Reporting
Effort reporting is the federally-mandated process by which the salary charged to a sponsored project is certified as being reasonable in relation to the effort expended on that project. Each academic institution establishes a process for effort reporting and the documented policies and procedures must meet the federal standards. Effort reporting is usually done on an annual basis, but it is important to understand the concept so that salaries can be apportioned appropriately throughout the year.
6.2.8 Conflict of Interest
Each academic institution will have a Conflict of Interest (COI) policy and will construct a management plan when a conflict is disclosed. An actual, potential, or appearance of conflict between the personal interests of the individual and the University or the public are addressed by a University Board or central administrative office. Financial conflicts of interest can arise in an ERC due to the involvement of industrial partners and the development of the innovation ecosystem. Refer to the Guidelines for Preparing Annual Reports and Renewal Proposals for specific reporting requirements and see Chapter 5, Section 5.3.4 of this Manual for further information.
6.2.9 Intellectual Property
ERC researchers work at the interface of discovery and innovation, and will therefore generate intellectual property and deal with technology transfer. Section 5.3.2 of the this Manual presents detailed information, but the Administrative Director should be prepared to work closely with the ERC’s Industrial Liaison Officer and the lead and core partner institutional offices to facilitate transactions such as:
- Documentation of Invention disclosures
- Licenses
- Patent process
- Materials Transfer Agreements (MTAs)
- Confidential Disclosure Agreements (CDAs)
- Intellectual property clauses for Sponsored Research Agreements
- Industry membership agreements.
6.2.10 Sub-recipient monitoring
Academic partners of the ERC will have subcontracts (or subawards) that outline the responsibilities of each party. These institutions may be called sub-awardees, subcontractors, or sub-recipients. The technical and programmatic section of the contract will detail requirements for progress reports, deliverables, and milestones. The financial section will specify dollar amounts and invoicing procedures, and the contract will always follow the institutional policies and terms of the NSF Cooperative Agreement. The AD should work closely with the Sponsored Programs office to make sure that the means of monitoring activities and measuring compliance are appropriate.