New Acid Catalysts Increase Efficiency in Refining Diesel-Range Fuels
Outcome/Accomplishment
New catalyst compositions are producing higher rates and better yield for diesel-range fuels in processes developed at Center for Innovative and Strategic Transformation of Alkane Resources (CISTAR), a U.S. National Science Foundation (NSF)-funded Engineering Research Center (ERC) based at Purdue University.
Impact/Benefits
These compositions and processes developed at NSF CISTAR show promise for advancing the refining industry’s use of catalytic processes. The processes are shown to convert olefins, another innovation studied at NSF CISTAR, at rates 500 times greater than traditional thermal reactions in producing diesel-range fuels.
Explanation/Background
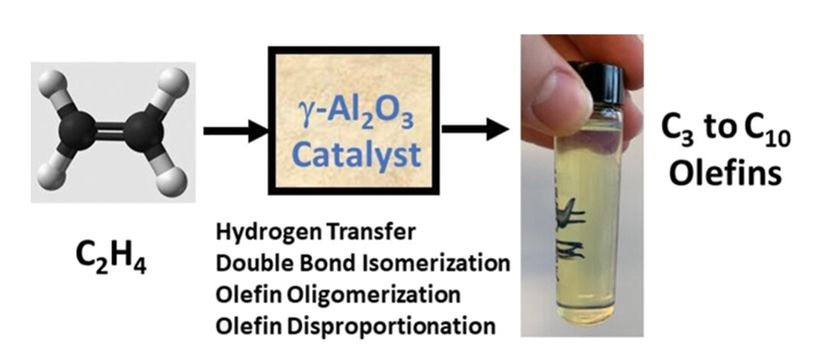
The research on new catalysts builds on NSF CISTAR’s study of olefins, or alkenes, a class of hydrocarbons used as building blocks for many products such as plastics. The new catalysts prove more efficient than thermal reactions in oligomerization, a reaction used to upgrade light olefins to heavier molecules useful as liquid fuels.
These newer “Lewis acid catalysts,” notably including alumina, are highly stable, showing little deactivation for more than a week. New catalytic reactions of olefins by Lewis acid catalysts produce gasoline- and diesel-range hydrocarbons by different chemistry than previously known Brønsted acid catalysts, such as zeolite, do. An initial evaluation of the process under realistic reaction conditions suggests the new catalysts are attractive for commercial development.
Location
West Lafayette, Indianawebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
New catalyst compositions are producing higher rates and better yield for diesel-range fuels in processes developed at Center for Innovative and Strategic Transformation of Alkane Resources (CISTAR), a U.S. National Science Foundation (NSF)-funded Engineering Research Center (ERC) based at Purdue University.
Location
West Lafayette, Indianawebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
These compositions and processes developed at NSF CISTAR show promise for advancing the refining industry’s use of catalytic processes. The processes are shown to convert olefins, another innovation studied at NSF CISTAR, at rates 500 times greater than traditional thermal reactions in producing diesel-range fuels.
Explanation/Background
The research on new catalysts builds on NSF CISTAR’s study of olefins, or alkenes, a class of hydrocarbons used as building blocks for many products such as plastics. The new catalysts prove more efficient than thermal reactions in oligomerization, a reaction used to upgrade light olefins to heavier molecules useful as liquid fuels.
These newer “Lewis acid catalysts,” notably including alumina, are highly stable, showing little deactivation for more than a week. New catalytic reactions of olefins by Lewis acid catalysts produce gasoline- and diesel-range hydrocarbons by different chemistry than previously known Brønsted acid catalysts, such as zeolite, do. An initial evaluation of the process under realistic reaction conditions suggests the new catalysts are attractive for commercial development.