CMaT’s Modeling and Analysis Tools Address Supply Chain and Process Concerns for Cell Therapy Manufacturing and Distribution
The Center for Cell Manufacturing Technologies (CMaT), headquartered at the Georgia Institute of Technology, developed several key resources to improve cell therapy manufacturing and distribution. The innovations at the National Science Foundation Engineering Research Center (NSF-ERC) included digital models of both a centralized and a distributed cell manufacturing and distribution network; a capacity planning tool that selects the optimal locations and capacities of manufacturing facilities; and a hybrid cost analysis model capable of activity-based costing and parametric costing to assess the cost implications and return-on-investment of cell manufacturing technologies.
A major barrier to the widespread adoption of CAR T-cell therapy is the unprecedented complexity of their manufacture and distribution, particularly with respect to the cell therapies’ personalized nature. Factors informing the commercial supply chain include short and predictable turnaround times for cell removal, modification, and return; high manufacturing success rate; increased efficacy and patient safety measures; and affordable treatment costs. Manufacturers must be able to collaborate efficiently end-to-end; manage schedules, volatility, and demand; optimize costs; and ensure patient safety and security. To meet these demands a digital supply chain is critical for allowing stakeholders to contribute and use data to keep track of the product, environmental conditions, and performance through each step of the treatment process. The novel supply chain and process modeling algorithms, methods, and tools developed by CMaT and its partners enable greater efficiencies in the supply chain, storage, and distribution systems for these therapeutic cell products.
The Georgia Tech Manufacturing Institute (GTMI) partnered with CMaT, the Marcus Center for Therapeutic Cell Characterization and Manufacturing (MC3M), the Institute for Data Engineering and Science, and BioFab USA to develop a customizable and interactive supply chain simulation program and decision support system to facilitate informed and dynamic supply chain modeling, tailored to the unique needs of the biomanufacturing industry. The project’s goal is to develop and validate digital models (for a single production facility and a network of production facilities) of manufacture and quality assurance for regenerative medicine and cells to support reliable, scalable manufacturing of quality, affordable therapeutics.
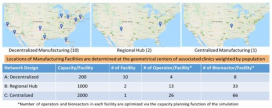
CMaT research led by Ben Wang included the design of a computation experiment for a comparison study of three supply chain network structures. Additional work investigated impacts of reagent supply disruptions and labor shortages on patient access and capacity utilization. Chimeric antigen receptors (CAR)-T cell therapies were used as an exemplar product. The team modeled response surfaces of patient mortality versus reagent availability under FIFO (First-In, First-Out) policy in a disruption duration of 9 months and completed case studies of cost analysis. Wang’s findings have been published in three journal articles appearing in American Pharmaceutical Review; Cell and Gene Therapy Insights; and Cytotherapy.
Summary results from the case studies revealed data on cost analysis, capacity planning, supply chain disruptions, demand surges and priority queue, cost of goods, and automation. Some of the unique challenges for managing the supply chain include real-time impact of patient health conditions on production and supply chain planning and the ability to address uncertainties like demand fluctuations, machine breakdowns, process failures, and supply chain disruptions. To date, the modeling has been informative for production capacity planning; risk mitigation strategies addressing supplier disruptions; and policy evaluations in response to demand surges. Visualized data reveals the impact of different gene-transfer methods on CAR-T cost and the impact of automation on iPSC cost.