Personalized Healthcare, One Cell at a Time
Cell engineering for personalized therapies is moving closer to reality with research advances that enable selection of cells by their function. An interdisciplinary team from the University of California-Los Angeles (UCLA) developed arrays of magnets to isolate magnetically tagged cells, observe them, and then selectively release one based on time-dependent behavior. This work is being supported by the NSF-funded Engineering Research Center for Translational Applications of Nanoscale Multiferroic Systems (TANMS), headquartered at UCLA.
Multiferroics are materials that exhibit more than one of the primary ferroic properties in the same phase, including ferromagnetism, which is switchable by an applied magnetic field, and ferroelectricity, an electric polarization that is switchable by an applied electric field. TANMS’ mission is to engineer a revolution in miniature electromagnetic electronics through development of a new class of nanoscale multiferroic materials. Current cell sorting technologies use cell surface markers as a marker for cell function, observing them instantaneously during sorting processes instead of looking at cell function itself. The new research can enable selection of individual cells based on their behaviors or time-dependent functions (such as cell killing, secretion, or movement). The researchers showed the system was compatible with an assay used to characterize secreted molecules from T cells, a type of white blood cell that is vital to developing immunity to viruses and pathogens and that is being leveraged for new cancer cell therapies. This proof of concept lays the groundwork for large arrays of magnets to capture, observe, and selectively release cells with specific functions that are expected to lead to improved performance as a therapy.
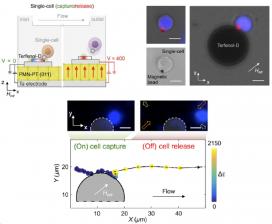
Programmable multiferroic materials present a potential technological transformation of cell sorting from bulk cell separation via an external magnetic field to single-cell separation via localized and programmable magnetoelastic micromagnets. Electromagnets at the scale of cells are too weak and consume too much power, so the TANMS research team looked to a new type of exotic magnetic material, Terfenol-D, which can maintain strong magnetic poles but can be switched intermittently with an electric field. The team was able to structure the Terfenol-D and show it could be formed into arrays of digitally switchable micro-magnets to control the capture and release of individual cells, a promising approach to enhance cell therapies.
The TANMS team first fabricated Terfenol-D microstructures at scales the size of a cell over the surface of a wafer. They learned that the structures possessed a single magnetic domain over a larger area than previous magnetic materials, leading to higher magnetic field strengths. The Terfenol-D microstructures enabled controlled trapping of magnetic beads with great precision. Magnetically labeled cells were then captured by the large magnetic field gradients (a variation in the magnetic field across space) generated from the single-domain microstructures. The magnetic state on these microstructures was switched by applying voltage to another material adjacent to the Terfenol-D micro-magnets. The electric field caused that material to deform and therefore modulated the magnetic state of the Terfenol-D micro-magnets, releasing individual cells.