Cell Sorting: Arrayed Single Cell Capture and Release Using Localized Oersted Fields
Researchers with the National Science Foundation’s (NSF) Translational Applications of Nanoscale Multiferroic Systems (TANMS) Engineering Research Center (ERC) at the University of California, Los Angeles (UCLA) have designed, fabricated, and tested a compact device that leverages magnets and fluids to selectively capture and release individual cells, enabling their study on a miniature chip. The device captures specific cells, which are tagged with magnetic beads, and securely immobilizes them for extended periods of examination or processing for disease fighting capabilities. The groundbreaking technology holds immense potential in the field of personalized medicine, particularly in the context of harvesting cells from an individual. By identifying supercells—cells with exceptional disease-fighting capabilities—this technology can aid in developing preventive treatments for diseases like cancer.
The study introduces a groundbreaking magnetic platform designed to capture a substantial quantity of clinically relevant cells, irrespective of their specific types, while ensuring the maintenance of cell viability. Unlike other devices that exclusively target particular cell types, often compromising cell health or imposing limitations on the number of cells that can be analyzed, this versatile platform overcomes such constraints. The platform has the capability to simultaneously process thousands of cells, utilizing layouts reminiscent of those found in magnetic memory storage, thereby accommodating terabytes of information. By facilitating effective single-cell analysis, this platform holds immense potential for driving the progress of next-generation therapies and making significant contributions to cancer and neurobiology research.
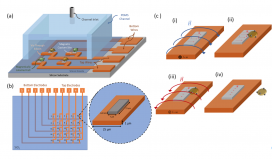
Understanding the nuances of individual cells, referred to as single-cell heterogeneity, holds immense importance in various research domains, including cancer, stem cells, and neurobiology. Analyzing single cells offers valuable insights that might remain concealed when studying cell groups collectively. To enable effective analysis, researchers need to isolate individual cells. Although methods like flow cytometry and microfluidic devices are available for this purpose, existing devices often have limitations in processing a large number of cells to identify a sufficient number of supercells required for personalized medical treatments. To overcome this challenge, a groundbreaking microfluidic platform array has been developed that leverages magnets to capture and release single cells in a controlled manner, thus surpassing the limitations of previous approaches. The functionality of the device relies on the utilization of minuscule magnets that can be switched on and off through the application of electric pulses. The layout of this platform resembles computer magnetic memory arrays, with dimensions tailored to the individual cells of interest. This breakthrough in scaling up opens up exciting possibilities for the development of personalized medical treatments.
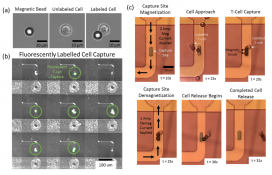
In experimental trials, the TANMS team successfully captured and released arrays of both magnetic beads and labeled Jurkat cells individually. The platform represents a significant breakthrough in single-cell analysis, surpassing the limitations of large array structures that lack individual cell study capabilities. By integrating magnetic capture sites and employing a sophisticated system of localized magnetization and demagnetization, TANMS’ platform offers unparalleled versatility in capturing and releasing magnetically labeled cells. Furthermore, the capability to immobilize captured cells for extended periods and the scalability of the device for high-throughput analysis greatly enhances its potential to revolutionize research fields such as cancer, stem cell biology, and neurobiology.